Johnson & Johnson will get FDA approval for coronary heart therapies that do not require X-rays
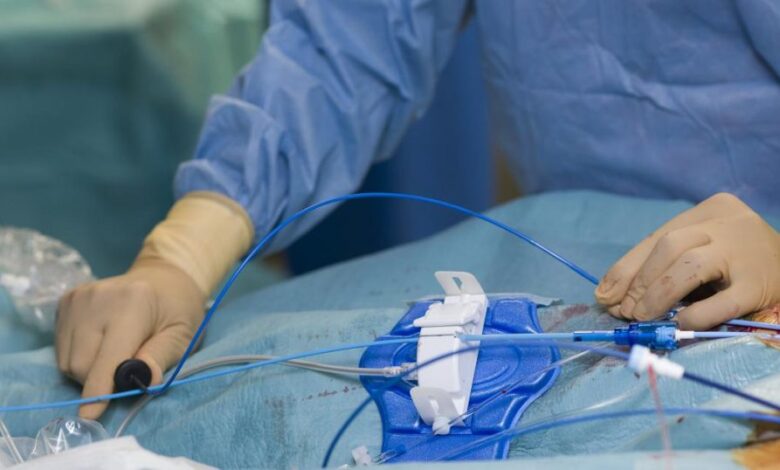
Johnson & Johnson’s acquired that can make it safer for medical professionals to deal with atrial fibrillation, a situation that makes your heartbeat irregular and might . A number of merchandise developed by Biosense Webster, which is a part of J&J MedTech, bought the OK for a “zero fluoroscopy workflow” from the FDA, that means dwell X-ray imaging will now not be wanted throughout catheter insertion procedures. As a substitute of utilizing X-rays to insert Biosense catheters, medical professionals can now use ultrasound to information therapies.
Utilizing fewer X-rays, or fluoroscopy, lowers radiation publicity for each sufferers and medical professionals. At present, docs and medical employees who work in therapy rooms focusing on treating related coronary heart procedures typically get an excessive amount of publicity to radiation over time, which may result in issues like eye points, most cancers, and bone accidents. This FDA approval helps handle the recurring occupational hazard. Suppliers working in cath labs additionally will not must put on heavy protecting gear like lead aprons anymore when making use of the newly authorized workflow, decreasing the chance of long-term muscle and bone ache.
This transfer by the FDA marks the primary and solely approval of its sort. The thumbs up was based mostly on knowledge from medical trials and analysis from the REAL AF Registry, or the real-world proof registry within the electrophysiology discipline. The information backed how effectively the therapy works in real-life conditions. The brand new methodology will solely apply for Biosense merchandise just like the , probably the most generally used ablation catheter, amongst others.
#Johnson #Johnson #FDA #approval #coronary heart #therapies #dont #require #Xrays





